Sign in with
Forget Password?
Learn more
share this!
Share
Tweet
Share
Email
November 21, 2025
by Perelman School of Medicine at the University of Pennsylvania
edited by Sadie Harley, reviewed by Robert Egan
scientific editor
associate editor
This article has been reviewed according to Science X’s editorial process and policies. Editors have highlighted the following attributes while ensuring the content’s credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
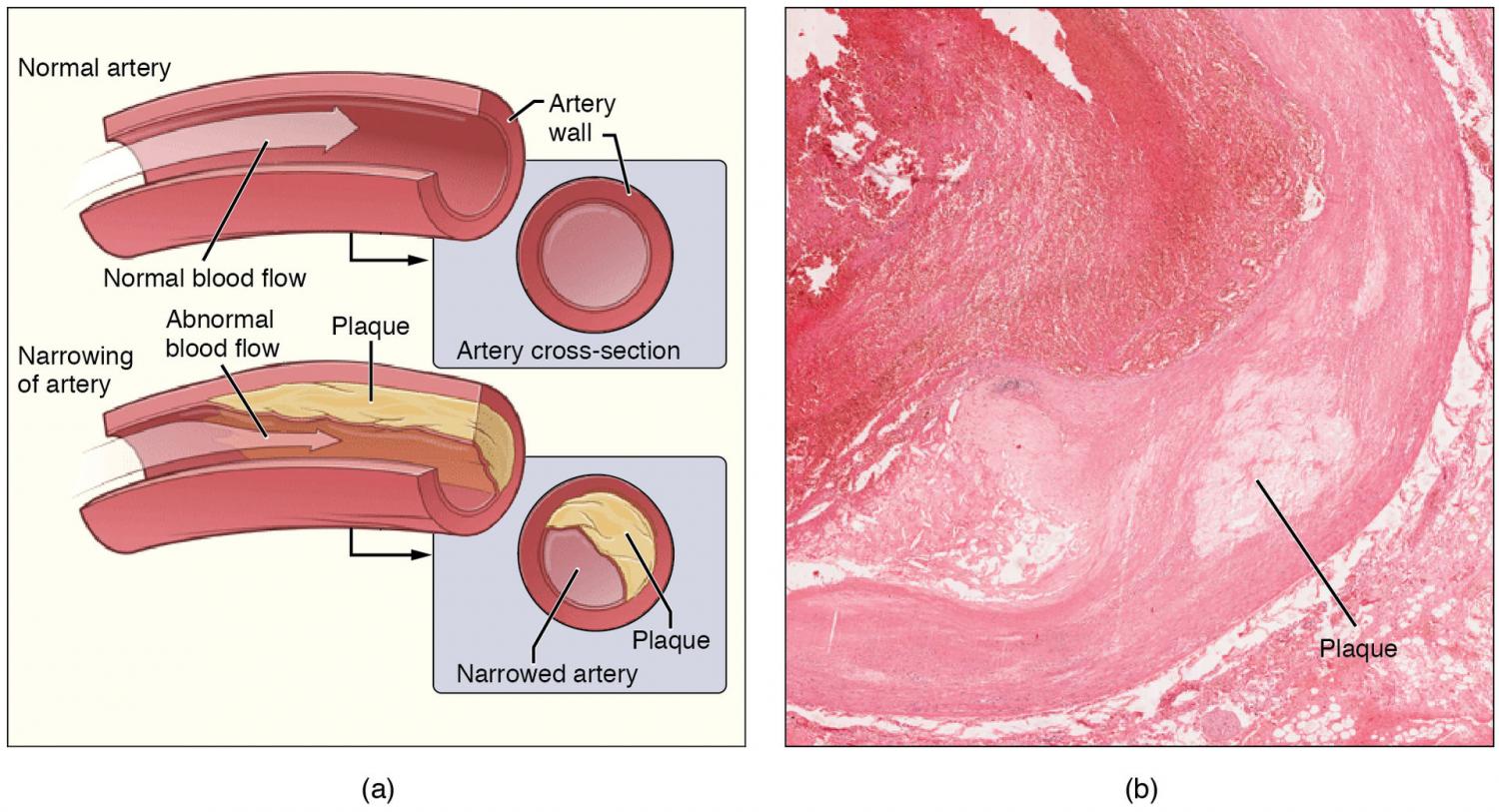
A pioneering preclinical study has shown that CAR T cell therapy—a personalized form of immunotherapy used in cancer treatment—could be a highly effective tool against atherosclerosis, the condition where a build-up of plaque in the arteries reduces blood flow, leading to heart attacks and strokes.
In tests in mice, the experimental CAR T cells blocked inflammation in arteries, preventing more than two-thirds of the plaque buildup seen in untreated controls. The research, led by scientists in the Perelman School of Medicine at the University of Pennsylvania, was published in Circulation.
“Our study shows for the first time how CAR T cell technology could be used to treat the underlying cause of the most common form of heart disease, which is the leading cause of death worldwide,” said senior author Avery Posey, Ph.D., an assistant professor of Pharmacology.
“This preclinical finding represents an important step forward for continuing to expand the impact of CAR T cell therapy to common diseases beyond cancer.”
Atherosclerosis underlies ischemic heart disease and stroke, which together kill tens of millions globally each year. Available treatments include medications to reduce low-density lipoprotein (LDL) cholesterol, which causes the buildup of plaque, and lifestyle modifications to reduce the risk of disease.
Although atherosclerosis is largely driven by inflammation, there are no approved treatments that specifically target atherosclerotic inflammation, and previous clinical trials of anti-inflammatory drugs have not panned out.
“The idea that therapy targeted to inflammation within the arterial wall can reduce the risk of atherosclerotic cardiovascular disease is compelling,” said co-author Daniel J. Rader, MD, an expert on lipids and atherosclerosis and chair of the Department of Genetics.
“The use of a CAR T approach to target the proinflammatory molecule oxLDL could provide an important complementary treatment approach to reducing the high residual risk of atherosclerotic cardiovascular disease in patients on effective cholesterol-lowering therapy.”
Researchers do not anticipate that CAR T cell therapy, should the approach prove successful in clinical trials, would replace existing treatments when they’re working well for patients. Rather, it could become another tool for patients who need additional or alternative treatment options.
CAR T cell therapy has revolutionized treatment for blood cancers. It works by engineering a patient’s own T cells in the lab and training them to recognize a marker found on cancer cells, creating an immune response that destroys the cancer.
Scientists have been exploring the potential of this powerful technology to treat other diseases, including autoimmune diseases and cardiac fibrosis, another form of heart disease. In this study, the researchers used a different type of T cell called regulatory T cells (Tregs), the subject of the 2025 Nobel Prize in Physiology or Medicine. Tregs dampen—rather than incite—the activity of other immune cells nearby.
The team engineered a CAR Treg that targets oxidized LDL (OxLDL), the main inflammation-stoking form of LDL cholesterol that drives plaque buildup in atherosclerosis.
“OxLDL is a pro-inflammatory molecule, and that inflammation is what starts atherosclerosis,” explained lead author Robert Schwab, MD, an instructor of Hematology-Oncology.
“The idea was, if we can get the immune system to see OxLDL and provoke an anti-inflammatory response, it would reduce inflammation and essentially stop the pathogenesis in its tracks.”
Initial lab-dish tests with human cells confirmed that the anti-OxLDL CAR Tregs suppress inflammation in response to OxLDL, greatly reducing the buildup of the cells that are a central feature of atherosclerotic plaques.
The team then engineered a mouse version of the anti-OxLDL CAR-Treg and tested it in mice that were genetically predisposed to high cholesterol and atherosclerosis.
After about twelve weeks of treatment, the treated mice’s hearts and aortas showed a roughly 70% lower atherosclerotic plaque burden compared to control mice—indicating a clear preventive effect of the CAR-Tregs. Despite this effect, there was no disruption of general immune function in the treated mice.
The researchers and Penn have founded a spinout company, Cartio Therapeutics, to continue developing the OxLDL CAR Tregs to test the therapy in human clinical trials.
Both Posey and Schwab trained under CAR T cell therapy pioneer Carl June, MD, the Richard W. Vague Professor of Immunotherapy, who led the development of the first CAR T cell therapy, approved by the FDA in 2017.
Posey has continued his independent CAR T research career in Penn’s Center for Cellular Immunotherapies, which is directed by June, and Schwab continued his medical training as an oncologist, completing a fellowship in the division of Hematology-Oncology at Penn earlier this year.
“Cancer, inflammation, and heart disease go hand-in-hand,” Schwab said. “It’s a real shame to see a patient cured of cancer die from a heart attack a year or two later.”
For many patients who survive cancer, heart disease becomes their biggest health risk, in part due to known side effects of cancer treatments and in part due to the cancer itself creating an inflammatory environment where diseases like atherosclerosis can thrive.
“We’re inspired by the potential that this technology developed for cancer could have to help so many people, cancer survivors included,” Posey said.
More information: OxLDL-Targeted Chimeric Antigen Receptor T Regulatory Cells Reduce Atherosclerotic Plaque Development, Circulation (2025). DOI: 10.1161/CIRCULATIONAHA.125.073987
Explore further
Facebook
Twitter
Email
Feedback to editors
20 hours ago
0
22 hours ago
0
22 hours ago
1
Nov 20, 2025
0
Nov 19, 2025
0
55 minutes ago
4 hours ago
15 hours ago
16 hours ago
16 hours ago
16 hours ago
16 hours ago
17 hours ago
17 hours ago
17 hours ago
Jun 2, 2025
Nov 13, 2024
Nov 13, 2024
Apr 30, 2024
Aug 20, 2025
Dec 2, 2024
21 hours ago
18 hours ago
22 hours ago
Nov 19, 2025
Nov 19, 2025
Nov 19, 2025
Engineered CAR T regulatory cells targeting oxidized LDL significantly reduced arterial plaque and inflammation in a mouse model of atherosclerosis, achieving about 70% less plaque compared to controls without impairing general immune function. This approach may offer a novel immunotherapy for atherosclerosis, complementing existing cholesterol-lowering treatments.
This summary was automatically generated using LLM. Full disclaimer
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form. For general feedback, use the public comments section below (please adhere to guidelines).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient’s address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we’ll never share your details to third parties.
More information Privacy policy
We keep our content available to everyone. Consider supporting Science X’s mission by getting a premium account.
Daily science news on research developments and the latest scientific innovations
The latest engineering, electronics and technology advances
The most comprehensive sci-tech news coverage on the web