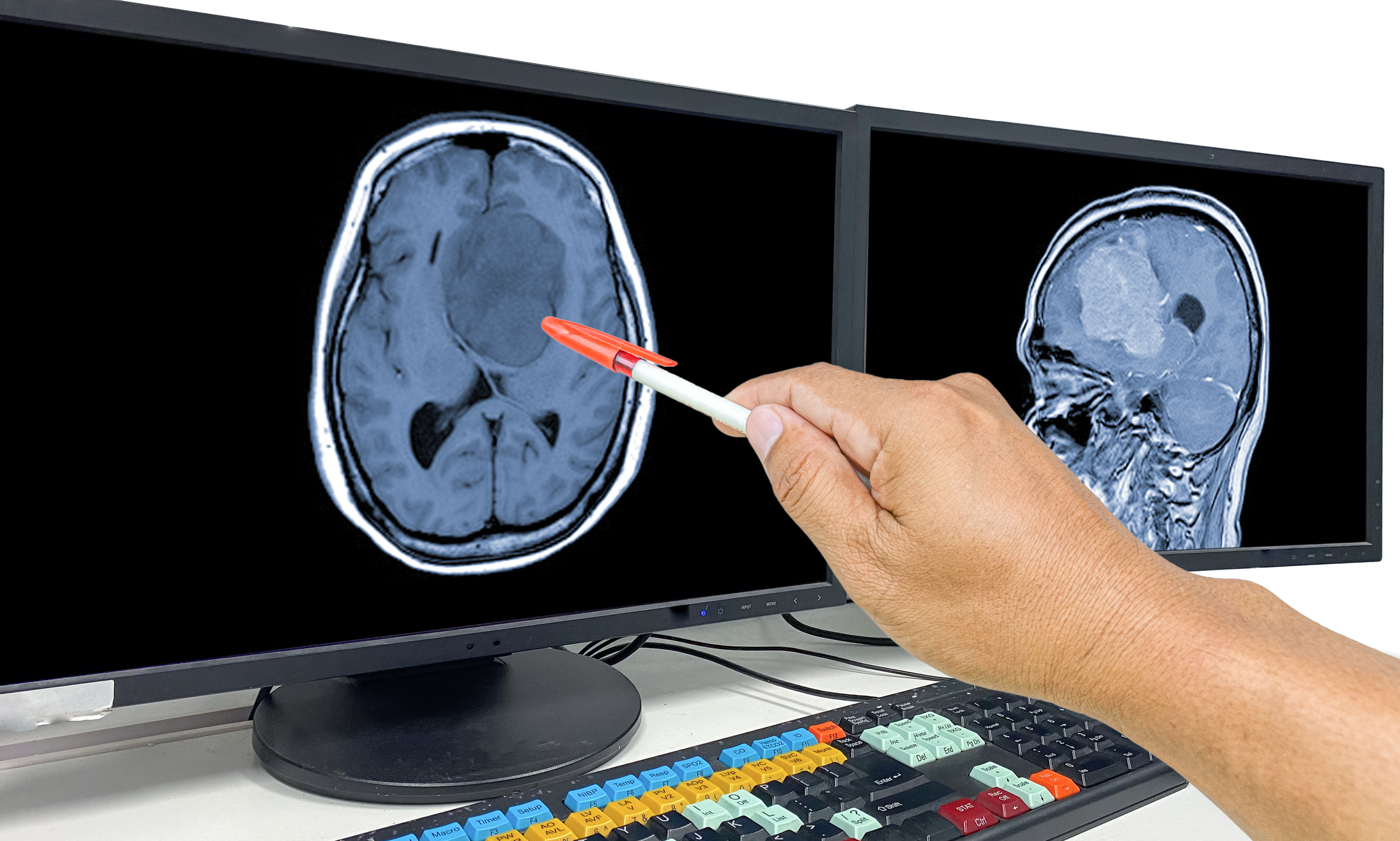
Scientists at the University of Geneva (UNIGE) and the Geneva University Hospital (HUG) have engineered a new CAR T cell therapy approach that can destroy glioblastoma cells. The new lab-engineered therapy, details of which are published in the Journal for ImmunoTherapy for Cancer, targets Tenascin-C (TNC), a protein secreted in the extracellular matrix by glioblastoma cells that is found within the glioblastoma tumor microenvironment. In preclinical trials, these CAR T cells induced tumor regression showing their potential as a new method to address this hard-to-treat brain cancer.
“Glioblastoma presents as a mass in the brain, consisting of tumor cells but also other types of cells, as is the case in most cancers. However, glioblastoma is unique in that it contains very few T cells, the immune cells that are able to recognize cancer cells and destroy them,” said study co-author Valérie Dutoit, PhD, a researcher in the department of medicine and the Translational Research Centre in Onco-Haematology (CRTOH) in the UNIGE Faculty of Medicine. “This is why glioblastoma, unlike melanoma or certain lung cancers, for example, does not respond to standard immunotherapies. Our approach is therefore to provide the patient with the missing T cells by generating them in the laboratory.”
Standard therapies for glioblastoma include surgery, radiotherapy, and chemotherapy produce only short-term responses. Immunotherapies such as immune checkpoint inhibitors, oncolytic viruses, and tumor vaccines to address this cancer have so far failed to produce meaningful clinical benefits.
Developing glioblastoma therapies faces multiple barriers. The tumor is highly heterogeneous, the brain is sensitive to off-target effects, and the immune-suppressive tumor microenvironment reduces CAR T cell efficacy. Moreover, conventional CAR T cells for solid tumors often face rapid exhaustion and limited persistence.
Prior work by the UNIGE-HUG team had identified the protein PTPRZ1 as a promising tumor surface marker for CAR T cell therapy. “In a previous study, we identified an important target, the PTPRZ1 marker, which is present on the surface of certain tumor cells. However, attacking glioblastoma on a single target is not enough to avoid the risk of relapse,” said senior researcher Denis Migliorini, MD, professor in the department of medicine at UNIGE and head of neuro-oncology at HUG.
Based on this, the researchers developed CAR T cells that target TNC, which provides structural support and modulates immune responses within the tumor microenvironment. By directing CAR T cells against TNC, the therapy triggers pro-inflammatory reactions that induce death not only in TNC-producing cells but also in neighboring tumor cells that do not express TNC, amplifying its antitumor effect.
The team tested the TNC-CAR T cells in vitro on patient-derived glioblastoma cell lines grown as adherent cultures or neurospheres, as well as with a purified TNC protein in ex vivo tumor samples. In both instances the CAR T cells showed antigen-specific activation, proliferation, and cytotoxicity, including bystander killing of TNC-negative cells when adjacent to TNC-secreting cells. In vivo testing in mice with patient-derived glioblastoma tumors showed efficient tumor infiltration, cancer cell apoptosis, and extended survival without detectable levels of off-tumor toxicity, the researchers noted. This finding corroborated the team’s work in preclinical models that showed intracranial administration of TNC-CAR T cells did not produce neurological symptoms.
The researchers also noted that this CAR T approach could also be effective in combination treatment strategies for glioblastoma. TNC levels have been shown to increase after radiation therapy which leads to treatment resistance, so pairing these CAR Ts with radiation therapy could significantly delay the development of resistance.
Because glioblastoma is highly heterogenous, the team not intends to further develop CAR T cells that can target multiple tumor antigens, while also looking to improve ways to prevent T cell exhaustion. The results also point to in-human studies with the researchers planning to launch a Phase I clinical trial in 2026.
Join host Jonathan D. Grinstein, PhD, North American Editor for Inside Precision Medicine, as he uncovers the stories behind the pioneers driving the precision medicine revolution.
Stay up to date with the latest episodes of Behind the Breakthroughs by subscribing to the IPM eNewsletter.
A contributing partner:
Copyright © 2025 Sage Publications or its affiliates, licensors, or contributors. All rights reserved, including those for text and data mining and training of large language models, artificial intelligence technologies, or similar technologies.