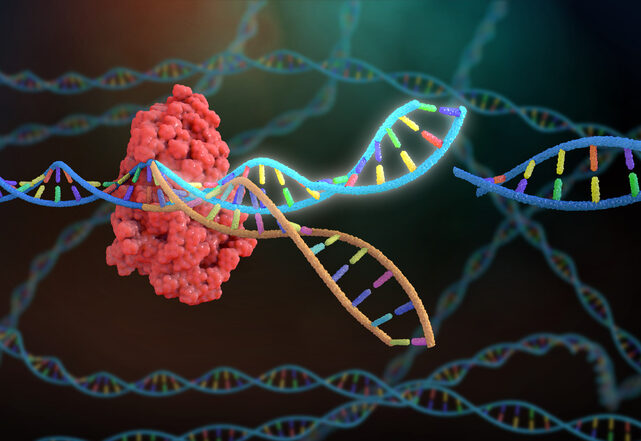
Researchers at the CeMM Research Center for Molecular Medicine of the Austrian Academy of Sciences and the Medical University of Vienna have developed a new method to systematically boost the therapeutic performance of CAR T cells for the treatment of certain blood cancers. The study, published in Nature, describes their tool called CELLFIE (CRISPR Engineered Library for Functional Interrogation and Enhancement), a high-content CRISPR screening and CAR T-cell engineering method that systematically identifies genetic modifications to improve the performance of CAR T cells for treating cancer.
“Our CELLFIE platform tests knockouts of all human genes in parallel and assesses which ones make CAR T cells fitter, more persistent, or less exhausted,” said first author Paul Datlinger, PhD, associate director of genome engineering, virtual cell initiative at the Arc Institute in California.
CAR T cells are created by engineering a patient’s own T cells with a chimeric antigen receptor (CAR), which enables them to recognize and destroy cancer cells. But one shortcoming of how these cells are created is they do not benefit from natural evolutionary optimization. “Unlike natural T cells, which evolved over millions of years, CAR T cells are genetically equipped with a new function, but evolutionarily not optimized for it,” the researchers wrote.
So, while CAR T therapies have been highly effective in some cases in treating blood cancers, they often fail for a variety of reasons such as T-cell exhaustion, poor proliferation, or immune suppression in the tumor environment.
To discover how CAR T-cell performance could be improved, the CeMM team performed 58 genome-wide CRISPR knockout screens in human primary CAR T cells using the CELLFIE platform. These screens assessed characteristics that could improve CAR T therapy effectiveness such as T-cell proliferation, target recognition, activation, apoptosis, and exhaustion. The researchers then prioritized gene edits using a new in vivo pooled CRISPR screening method in mouse models of leukemia.
Using this method, the team discovered a surprising target, the RHOG gene. When the researchers knocked out RHOG genes in preclinical models of leukemia, CAR T cells became substantially more potent.
“(RHOG) plays a crucial role in our immune system but reduces the effectiveness of CAR T cells. By knocking this gene out with CRISPR technology, we were able to increase the therapeutic potential of CAR T cells substantially,” said Eugenia Pankevich, co-first author and PhD candidate at CeMM.
The researchers then validated RHOG knockout in multiple CAR designs, tested against mouse models, and T-cell donors, including patient-derived cells. RHOG-deficient CAR T cells showed improved expansion, reduced exhaustion, and stronger tumor control compared to current CAR T-cell therapies.
The team also identified the FAS gene as another important target in CAR T-cell design. When FAS was knocked out, it enhanced CAR T-cell survival by reducing fratricide and apoptosis. Knocking out both the RHOG gene and FAS gene combined their effects. “By targeting both RHOG and FAS, we saw strikingly synergistic effects—the gene-edited CAR T cells proliferated faster, stayed more active, were less likely to kill each other, and were able to cure mice from aggressive leukemia,” said co-first author Cosmas Arnold, PhD, a project manager at CeMM.
This modular approach for CAR T-cell engineering using CRISPR editing could eventually be used to create tailored cell therapies. The CELLFIE platform also addresses major challenges in scaling and cost of genome-wide screens. The researchers developed an adapted version of their CROP-seq method for in vivo screening, allowing pooled validation of dozens of gene edits using only a fraction of the animal models currently needed.
Earlier studies have suggested the potential for this gene editing approach to optimizing CAR T-cell therapies. Genetic disruptions of immune checkpoints like PD-1 and CTLA4, or regulators like PRDM1 and RASA2, had already shown that CAR T-cell performance can be enhanced through targeted editing.
“Our study establishes an exciting candidate for future clinical validation as a therapy for certain blood cancers,” said principal investigator at CeMM Christoph Bock, PhD, who is also a professor at the Medical University of Vienna. “And we created a broadly applicable method for the systematic enhancement of cell-based immunotherapies. We are learning how to program cells as effective cancer therapeutics and as ‘living medicines’ for a wide range of diseases.”
With this new discovery, the team will next look to conduct additional preclinical testing of RHOG and FAS double knockouts, refine base-editing approaches for clinical safety, and explore the application of CELLFIE to other cell-based therapies, including those for solid tumors and autoimmune diseases.
Join host Jonathan D. Grinstein, PhD, North American Editor for Inside Precision Medicine, as he uncovers the stories behind the pioneers driving the precision medicine revolution.
Stay up to date with the latest episodes of Behind the Breakthroughs by subscribing to the IPM eNewsletter.
A contributing partner:
Copyright © 2025 Sage Publications or its affiliates, licensors, or contributors. All rights reserved, including those for text and data mining and training of large language models, artificial intelligence technologies, or similar technologies.